The huge amounts of data that laboratories in every sector of industry generate are a key asset for an organization. Maximizing value from that asset is, however, a major challenge. Today’s laboratory environment is commonly stacked with many different types of instruments and software from multiple vendors. This is a serious problem when attempting to coordinate the management, storage, access and analysis of disparate data that is generated across the laboratory IT ecosystem.
Seamlessly connecting processes and data in the lab is a primary endpoint of the Lab 4.0 concept, a vision for the lab-of-the-future that embodies digital transformation and automation. Such a transformation will enable labs to automate their processes and procedures, and digitally capture the flow of contextually relevant data from – and communication between – fully integrated, easily interfaced platforms in the lab and enterprise wide.
This drive for end-to-end automation and integration will likely be a long haul. Laboratory hardware and software from different vendors typically speak different ‘languages’, and so can’t easily be interconnected as part of a unified lab infrastructure. Most labs will have little or no chance of attaining a cohesive ‘plug-and-play’ setup or data management architecture, based on the equipment and software that they currently run.
Punctuated processes and workflows that result from the inability to integrate and automate mean that labs still rely on manual data entry, results recording, and paper-based processes. Manual transcription is inevitably liable to errors, and can necessitate data transfer via emailed ‘flat’ files – Word documents, PDFs and Excel spreadsheets – which are easily lost or buried in siloes, and have little or no accompanying contextual data or metadata. Such data is also unlikely to be in standardised format, and thus can’t be easily searched or compared. And being neither in the right format, complete, nor clean means that it can’t be used to train the next generation of machine learning (ML) algorithms and AI tools.
The benefits of a digitally interconnected, fully integrated laboratory are thus evident. Through ‘digital continuity,’ robotic systems, analytical instruments and software tools can communicate with each other to schedule, instigate and carry out workflows, generate and send results and reports, and validate processes and SOPs. Digital data can be routed to data lakes in real time and in findable, readable and insightful formats, with no loss of context, and with connection to related workflows and key metadata.
A digitally transformed laboratory could feasibly do away with the need for manual data entry, removing transcription errors, and automating all aspects of the lab operation – from sample receipt and inventory management, to scheduling, personnel training, authorisation and even instrument calibration.
By helping to ensure that all required activities are properly tracked and documented, and readily available for audits and investigations, digital transformation does not just make lab operations more efficient, it also improves regulatory compliance.
Keeping data in a digital format can help to ensure data integrity and support FAIR (findable, accessible, interoperable, reusable) principles for the derivation and management of scientific data. Digitally captured data ‘persists’ by the fact of it residing in an accessible digital format and, importantly, remains immediately available to the whole organization. The laboratory is also part of a greater ‘whole’, and so a lab 4.0-enabled enterprise will benefit from a centralised data management infrastructure, which will make all relevant data, from experimental results, inventory, analyses, and business intelligence, available across an organization.
The Lab 4.0 roadmap thus hinges on automation, integration, and connectivity, but the roadmap will be different for every lab, which will have its own starting point, priorities, and endpoints for that digital continuity journey. The use of augmented reality in the car design or manufacturing industry, for example, may not be so relevant in a pharmaceutical quality control laboratory or early-stage discovery environment.
Progress on any digital transformation journey will be based on a coordinated, collective review of the lab’s existing technology, its realistic goals and timelines. These goals should be evaluated alongside the human aspects of change management. The starting point of any digitalisation journey should thus be to review ‘what’ the lab is doing and ‘how’ it is doing it, with respect to the hardware and software that is already in place, and how it is used. Then it is possible to consider how key existing processes can be optimized digitally. Figure out what is important, and also, what is realistically achievable in the short, medium, and longer term.
Thinking forward, those individuals with the purchasing power – who likely will be neither the scientists and operators who work in the lab day-to-day, nor the lab managers – will have to consider not only whether new instrumentation and software will carry out the desired functions, but also whether it will sit well with the inevitable change management at a human level.
It’s a tall order for any organization, and so should involve collaboration between the scientists and technicians, lab managers, purchasing departments and decision-makers at the top. And ideally, vendors of the software and hardware platforms that are being considered should play a role, as consultants, in objectively helping potential customers to understand the technologies and how they will work within the existing setup.
Strip the digital transformation part of the lab 4.0 vision back to its bare bones, and the underlying aim is thus to enable integration, avoid manual transcription and data recording – and so transcription errors – and to have all laboratory data in standardised formats that can be searched, mined, compared, and analysed with full context, which will aid those ML and AI efforts.
Drive lab automation and integration, and the reduction, or potentially even complete removal, of manual transcription, will follow. Integration is typically one of the most immediate, but also far-reaching considerations when implementing a new platform such as a laboratory information management system (LIMS) or electronic laboratory notebook (ELN). The lab may have dozens of different types of devices, and potentially multiple devices of the same type – say, balances, or pH meters – but from different vendors, and getting all these hooked up to a LIMS, and talking standardised language, both for communication and instruction between instruments, and also for management of the results (say, mass in grams) and metadata (what was being weighed, where did it come from, which balance was used, who carried out the task, etc).
To enable this plug-and-play functionality and interconnectivity will require the widespread adoption of flexible standards for communication between instruments and software (the SiLA standard is one example) and also for data handling (AnIML, and Allotrope data formats are examples). When all vendors support standards in this way, device integration immediately becomes simplified, because the instruments and software ‘know’ in advance how to speak to each other, and how to handle data that is received and passed forwards.
While laboratory information management systems (LIMS) now commonly underpin the digital infrastructure in labs to manage samples, schedule tests and route results, many labs still do rely on paper-based processes, and so their digital journey will start at a more fundamental level. In addition to a LIMS, a modern laboratory execution system (LES) can further facilitate the orchestration of lab tasks day-to-day, remove the need for manual (paper-based) documentation of tasks, and so aid reproducibility, efficiency and compliance.
Whereas a LIMS user interface (UI) is typically designed for usage on a standard desktop or laptop PC, an LES platform should be optimized for usage on mobile touchscreen devices, so that it can be conveniently operated within a laboratory environment. The Starlims LES has been developed to allow lab technicians to easily record all parameters associated with their laboratory workflows, as an integrated stepwise, and validated sequence.
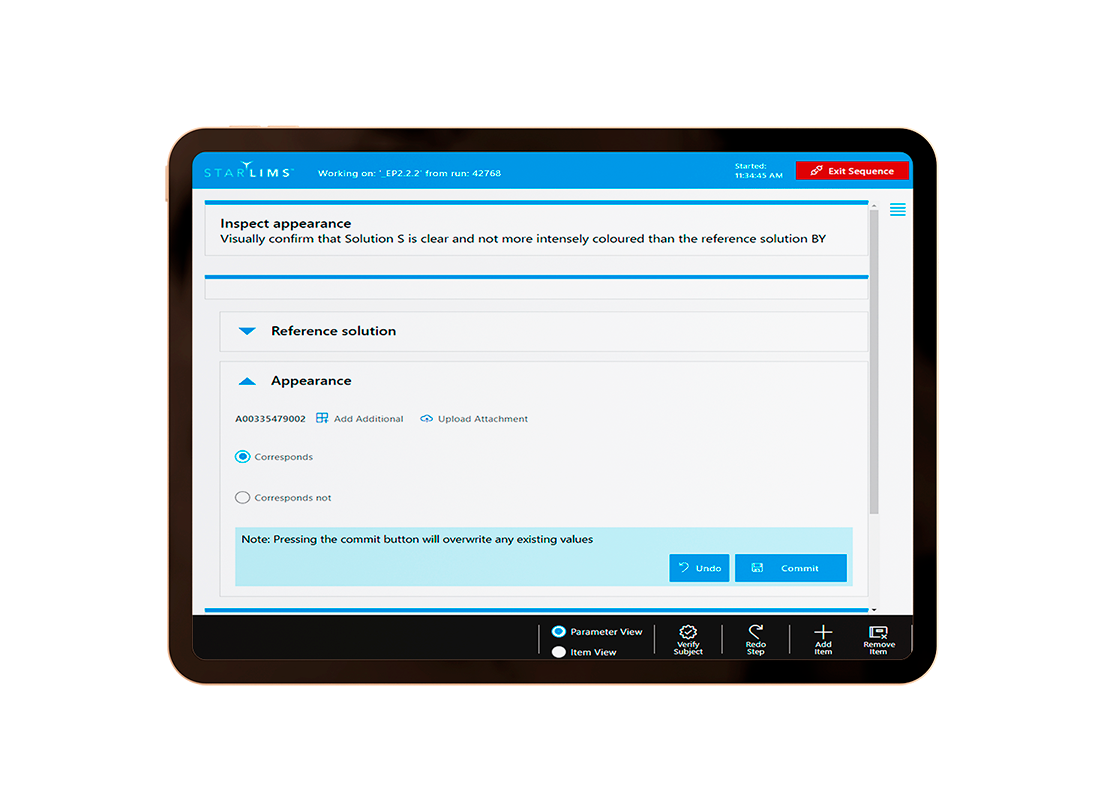
This workflow might be, for example, as simple as “weigh a sample, add solvent, then homogenize.” But the LES doesn’t just set the workflow in motion, it automatically prompts the technician to confirm actions, and then automatically records key parameters, such as (to use a previous example) which balance is being used, who is carrying out the work, which sample of solid is being weighed, and so forth. By barcoding sample containers, reagents and lab devices, information can be easily and reliably entered for those steps, thus minimizing the documentation overhead for the lab technicians. Required validation checks, such as confirming that reagents are not expired and that lab devices have been properly maintained and calibrated, can be done by the system automatically. All the information recorded in the Starlims LES are transferred back to the LIMS module, where they are available for analysis, reporting and review.
A LES can thus be an ideal complement to LIMS software, by giving lab technicians a powerful tool to document their work directly, with as little overhead as possible, and without the need for any paper-based protocols. Both efficiency and reliability can be improved by transferring data from lab devices like balances or pH-meters to the LES via validated interfaces, and by automatically running all required validation checks directly in the system.