Recherche Clinique

CRÉEZ UN IMPACT AVEC VOS TRAVAUX DE RECHERCHE
Il est vital que les laboratoires cliniques délivrent des résultats exacts. La solution STARLIMS Recherche Clinique est la garantie que les échantillons de votre laboratoire sont recueillis, manipulés et traités avec précision tout au long du cycle de vie de l’échantillon. STARLIMS évolue au rythme des besoins de votre laboratoire, offrant une flexibilité inégalée et la prise en charge des processus configurables par l’utilisateur. Cette solution s’adapte ainsi à un large éventail de projets de recherche, d’essais cliniques et de processus de laboratoire. Avoir confiance en votre capacité à répondre aux besoins changeants des chercheurs et aux réglementations toujours plus strictes, tout en fournissant des résultats de recherche exacts.
GESTION D’ESSAIS COMPLEXES DÈS LE DÉBUT
Le gestionnaire de tests STARLIMS offre aux utilisateurs la possibilité de définir et de configurer de nombreux paramètres, notamment :
- Protocoles d’étude
- Groupes de traitement
- Métadonnées du sujet/de l’échantillon
- Calendrier principal, y compris la planification des visites, les métadonnées des sujets spécifiques à la visite, les éléments de la visite, les limites de tolérance et les informations sur les séquences de visites
- Informations et vérifications relatives au consentement, conformément au conseil d’examen institutionnel (CEI) et aux exigences réglementaires, ainsi qu’aux préférences des sujets
- Utilisation de modèles et d’assistants pour établir des plages de référence, des réflexes, des déclencheurs et des exigences de reporting basés sur l’étude
SUIVRE ET MANIPULER FACILEMENT LES ÉCHANTILLONS
- Définir, identifier et localiser des échantillons dans un conteneur ou un lieu de stockage en utilisant des champs d’analyse à code à barres et des outils graphiques
- Repérer le lieu d’origine de l’échantillon, la date limite de renvoi et les personnes responsables
- Permettre aux chercheurs la possibilité de réserver les échantillons pour un intervalle de temps donné
- Ajouter de nouveaux attributs aux métadonnées d’un échantillon à utiliser lors des demandes de test
- Identifier et résoudre les divergences entre des attributs d’échantillon/du sujet (état à l’arrivée, conteneur, temps de transit, etc.) et les exigences des études cliniques
- Accéder à l’historique complet de l’échantillon, de l’ouverture du tube à la destruction de l’échantillon
GÉRER L’INVENTAIRE ET LE STOCKAGE DES ÉCHANTILLONS
- Gérer les processus de collecte, de réception, de préparation et d’analyse, de gestion du lieu de stockage, des demandes ainsi que le référencement des échantillons
- Afficher les données qui vous aident à gérer les stocks, l’utilisation et le mouvement des échantillons, l’affectation des analyses et bien plus encore
- Définir les composants d’un kit, la composition du porte-échantillons, les niveaux de stocks et l’envoi de kits grâce au gestionnaire de stocks STARLIMS et au gestionnaire de tests STARLIMS
- Planifier et suivre la capacité de chaque lieu de stockage afin de consolider les stocks et rappeler au personnel de réaliser des revues d’inventaire périodiques
UNIFORMISER ET AUTOMATISER LA LOGISTIQUE QUOTIDIENNE
- Automatiser l’importation des enregistrements électroniques, des demandes d’analyse et des fichiers de résultats
- Disposer d’une solution pour communiquer avec les outils de manipulation d’échantillons et des instruments d’analyse ; via des protocoles de communications comme les services Web, le transferts de fichiers (texte, CSV, HL7, ASCII, etc.) et les communications directes de base de données à bases de données
- Gérer la chaîne de responsabilité avec des signatures électroniques
- Gérer la facturation aux clients des services d’expédition, de traitement et d’analyses réalisés sur les échantillons
- Procéder à la commande, au conditionnement et à l’expédition grâce à l’outil de processus d’expédition
- Permettre aux chercheurs responsables d’essais cliniques, aux chefs de projet, aux fournisseurs de kits et aux laboratoires de référence externes de disposer d’un accès sécurisé au système
RESSOURCES UTILES
ANALYSES AVANCÉES EN ACTION !
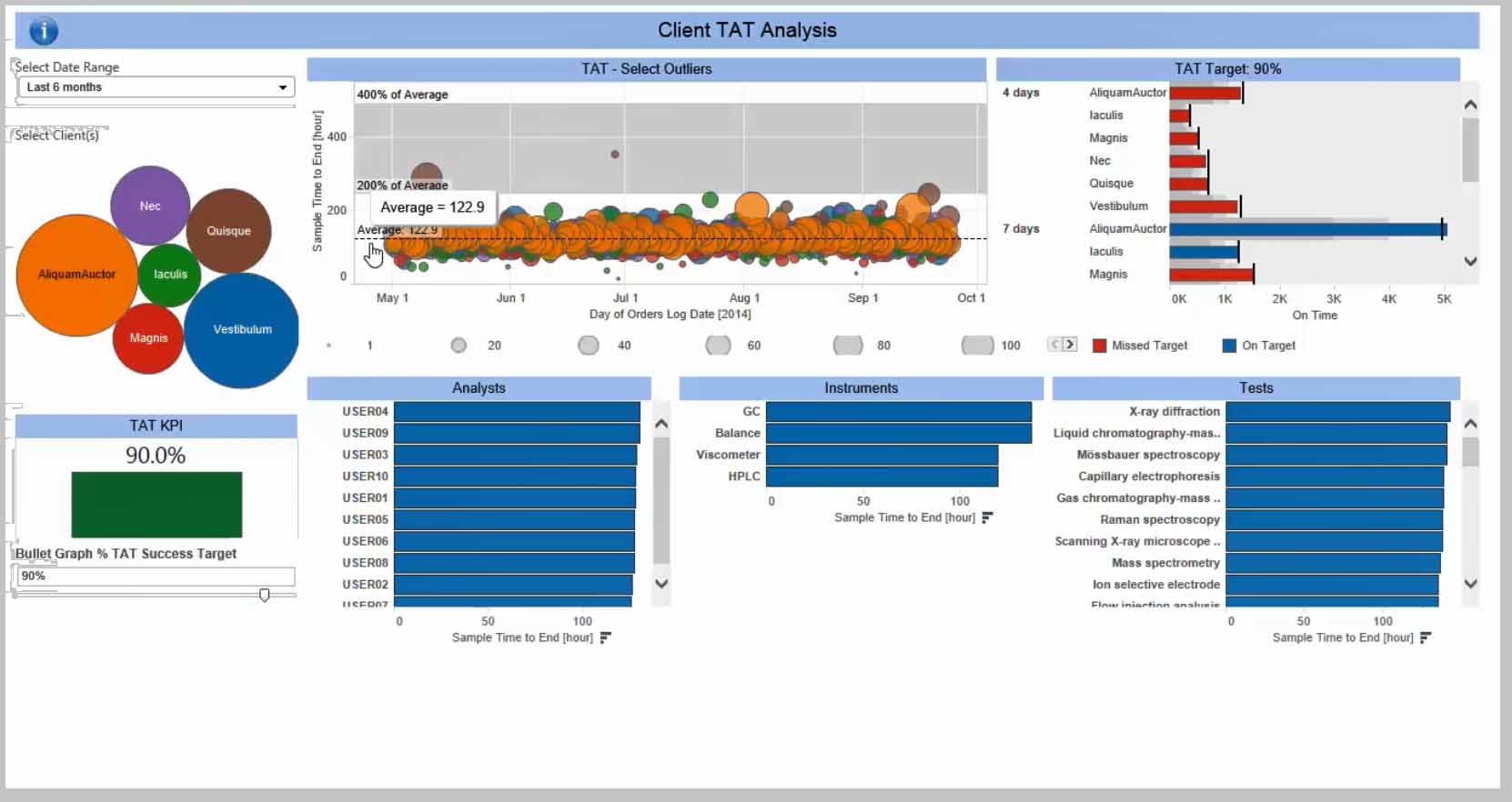
Délais d’analyse par client
Découvrir comment la solution Analyses Avancées peut permettre aux laboratoire de consulter au quotidien les délais d’analyse pour chaque client. Cette fonction fournit également des indicateurs de respect des délais, disponible au niveau des équipes, des instruments et des analyses.
AVIS CLIENTS
“La plupart des systèmes sont au même niveau en ce qui concerne les fonctionnalités. STARLIMS s’est distingué en proposant un système très ouvert avec une grande flexibilité et le meilleur mélange entre les fonctionnalités disponibles et la convivialité. De plus, nous avions confiance en leur équipe de projet.”
Alexandra Michel
Chef de projet, Bayer CropScience
“La solution LIMS est connectée à nos systèmes de production et de réception des matériaux au stockage. Cela signifie une avancée fondamentale dans les processus de contrôle de la qualité de l’entreprise, ainsi qu’un plus grand échange d’informations entre les systèmes.”
David Martínez Baños
Département informatique
“J’aime le fait qu’il soit convivial. J’aime le professionnalisme de tous mes interlocuteurs. J’apprécie également le fait qu’il s’agisse d’une amazon “STARLIMS gère de manière centralisée les échantillons et rationalise les processus de laboratoire associés pour nous permettre de fournir des données de qualité analytique en temps réel et a considérablement amélioré notre capacité à servir de point de contrôle de service pour tous les carburants aérospatiaux et les produits connexes pour les systèmes d’armement de l’AF dans le monde entier.”
Dave Fisher
Chef de la division laboratoire, Agence pétrolière de l'armée de l'air
“Le logiciel que nous utilisions auparavant était basé sur Microsoft Access. Avec toutes les limitations qu’il comportait, le principal problème auquel nous étions confrontés était l’impossibilité de l’interfacer avec d’autres logiciels (SAP, CRM, etc.).”
