Santé Publique

CONÇU POUR PROTÉGER LA SANTÉ DE LA POPULATION
Les autorités de santé publique du monde entier comptent sur la capacité de votre laboratoire à non seulement surveiller et suivre les pandémies et les épidémies pouvant survenir, mais également à déterminer les effets de causalité lorsqu’elles se produisent. La solution STARLIMS Santé publique automatise les processus tout au long du cycle de vie de l’échantillon, de la programmation et l’entrée de l’échantillon dans le système à l’établissement du rapport, permettant aux laboratoires de diffuser des résultats précis, en temps opportun, pour protéger la santé de l’ensemble de la population.
AVOIR CONFIANCE DANS LA CONFORMITÉ ET LES MEILLEURES PRATIQUES
- Respecter les directives et les meilleures pratiques du secteur. STARLIMS est partenaire de l’APHL, des Centres pour le contrôle et la prévention des maladies (CDC) et des établissements de santé publique dans plus de 30 laboratoires de santé publique régionaux, nationaux et internationaux pour s’assurer que notre logiciel vous permet de respecter les directives et meilleures pratiques du secteur
- Travailler avec un fournisseur certifié par le programme GSA Schedule 70. STARLIMS est certifiée par le programme GSA Schedule 70 pour la médecine légale et la santé publique. Voir toutes nos certifications
PRÉLEVER ET GÉRER LES ÉCHANTILLONS EN TOUTE TRANSPARENCE
- Collecter vos données sur le champ avec le Electronic Laboratory Notebook (ELN) (cahier de laboratoire électronique)
- Consigner les échantillons à l’avance et transmitter des informations les concernant avant de procéder à leur soumission, afin de réduire le personnel et le temps nécessaires pour accéder à un échantillon lors de sa réception
- Utiliser les codes-barres et des appareils portatifs pour générer des étiquettes pour échantillons et simplifier le suivi des échantillons à partir d’ appareils mobiles et en déplacement
ASSURER LA QUALITÉ DES RÉSULTATS DE LABORATOIRE
- Alerter automatiquement les analystes et les manageurs des échecs du contrôle qualité et des résultats suspects
- Configurer et créer des éléments déclencheurs dans le but d’aider votre personnel à suivre une procédure d’analyse et de manipulation définie par vos meilleures pratiques
- Guider les utilisateurs via des étapes prédéfinies qui respectent les procédures d’utilisation normalisées (SOP), en apportant aux utilisateurs des recommandations appropriées basées sur vos spécifications
UNIFORMISER ET AUTOMATISER LA LOGISTIQUE QUOTIDIENNE
- Prenez en charge les messages et les codes HL7, PHL, ICD-9, CPT, LOINC et SNOMED
- Réunir l’ensemble des résultats d’analyses et des documents justificatifs, indépendamment du format, dans STARLIMS Data Capture Utility (DCU)
- Intégrer l’ensemble de vos systèmes de gestion, de stockage et d’analyses de votre laboratoire dans un Scientific Data Management System (SDMS) (système de gestion de données scientifiques [SDMS] et un Electronic Laboratory Notebook (ELN) (cahier de laboratoire électronique [ELN]) aux fonctionnalités riches
LABORATOIRES DE SANTÉ PUBLIQUE ET LABORATOIRES DIAGNOSTIQUES SPÉCIALISÉS
Les tests moléculaires sont de plus en plus abordables et désormais plus courants. Même dans le domaine de la santé publique, les tests moléculaires deviennent la norme, et dans le cadre du diagnostic clinique, les tests moléculaires sont de plus en plus utilisés. Séquençage de nouvelle génération (NGS) devient de plus en plus important pour le profilage des maladies. La prolifération des tests moléculaires complexes tels que ceux de la NGS dans les laboratoires cliniques a conduit à la publication de nouvelles exigences et directives par les organismes CAP, CLIA et ACMG. Celles-ci couvrent le besoin de validation des tests et la gestion du traitement des échantillons, les données obtenues pendant les tests et la publication et la diffusion des résultats. Les inspecteurs du CAP et du CLIA commencent à se familiariser avec ces directives et le processus de recertification va devenir plus ténu. Les laboratoires qui ont mis en œuvre les nouvelles exigences et recommandations réduisent le risque de voir des déficiences constatées lors des inspections. Le fait de disposer d’un LIMS capable de démontrer une gestion efficace de ces processus moléculaires très complexes contribue à la croissance et à la validité du laboratoire.
Gérer efficacement les échantillons et les lots
- Gérer des protocoles moléculaires, dans lesquels les flux de travail existants peuvent être utilisés comme modèles pour de nouveaux protocoles, où les gammes de contrôle, les alertes et actions conséquentes, et les exigences en termes de rapports peuvent être configurés
- Prendre en charge les échantillons et le flux analytique de bout en bout, configurer les alertes, les points d’échantillonnage, les nouveaux échantillons et les flux de rééchantillonnage et de re-test
- Permettre l’ajustement par lot et l’utilisation de plusieurs modèles de lots dans un flux de travail complexe
- Évaluer les résultats des tests échantillons par rapport aux différents ensembles de spécifications et contrôler la progression de l’échantillon ou du lot à travers son cycle de vie
- Générer une feuille/liste de travail, des résultats calculés (formules de calcul) et appliquer des spécifications sur ces résultats
- Créer des interfaces avec des systèmes bio-informatiques (systèmes tiers) (internes ou externes) pour l’analyse des données avant l’émission du rapport de résultats
UNIFORMISER ET AUTOMATISER LA LOGISTIQUE QUOTIDIENNE
- Diriger les échantillons vers les bons équipements et les bonnes ressources en fonction des disponibilités, des habilitations du personnel.
- Interface vers des systèmes automatisés de gestion des échantillons et des instruments analytiques grâce à des communications de système à système utilisant une grande variété de protocoles tels que les services Web, le transfert de fichiers (texte, CSV, HL7, ASCII, etc.) et les communications directes avec les bases de données
- Gérer les rapports et la communication des résultats pour des échantillons uniques passant par plusieurs laboratoires/services et plusieurs flux de travail
- Gérer le stockage des échantillons tout en gérant les lieux de stockage
GESTION DES ÉCHANTILLONS ET DES ÉQUIPEMENTS
- Gérer tous les types d’échantillons/de matériaux moléculaires, les articles d’inventaire et les consommables ainsi que les flux de travail associés
- Gérer les listes d’équipements, les événements de maintenance programmés, les étalonnages et les échantillons de contrôle (QC)
- Stocker vos échantillons dans des conteneurs de stockage hiérarchisés et afficher le contenu à chaque niveau de la hiérarchie/arborescence de stockage
AVOIR CONFIANCE EN LA CONFORMITÉ AUX NORMES RÉGLEMENTAIRES ET DANS MEILLEURES PRATIQUES
- STARLIMS a été conçu pour aider votre organisation à se conformer à un large éventail de normes réglementaires de laboratoire différentes, notamment les directives FDA 21 CFR Part 11 et l’annexe 11 de l’UE, les BPL, APHL, CLIA, CAP et d’autres normes d’accréditation
RESSOURCES UTILES
ANALYSES AVANCÉES EN ACTION !
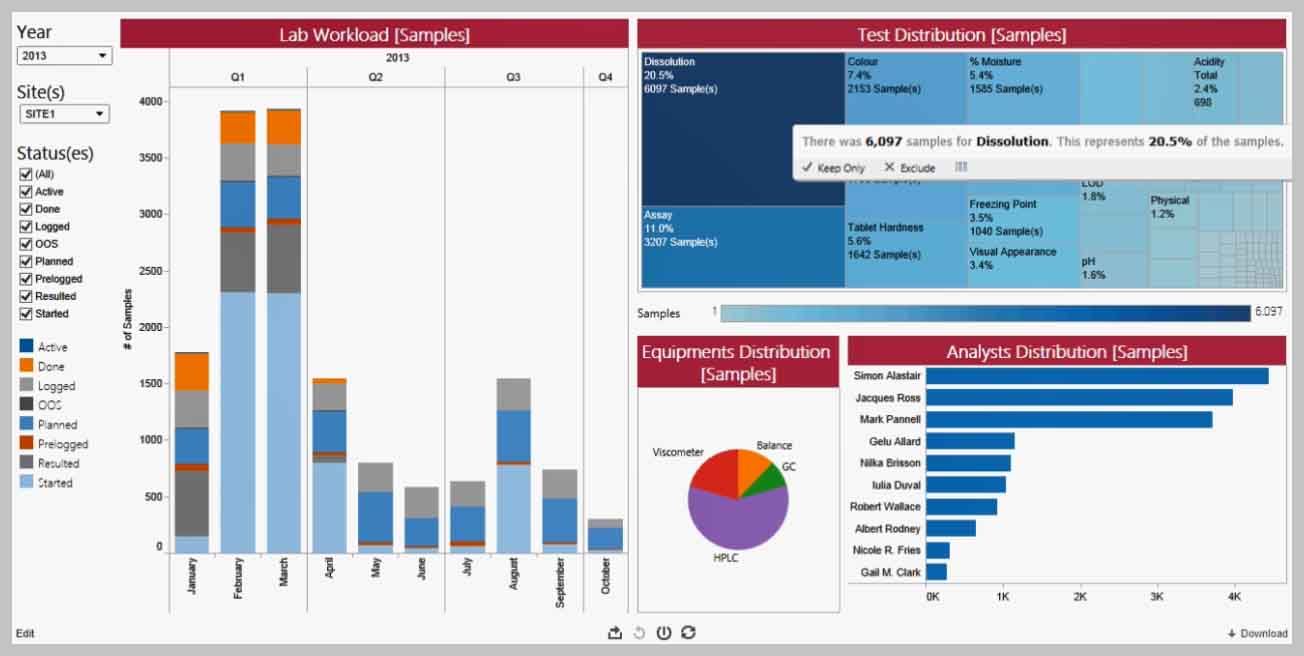
Charge de travail du laboratoire
Découvrir comment Analyse Avancée peut vous permettre de planifier votre travail en fonction des priorités du laboratoire, ou, quand les priorités changent, de comprendre les causes sous-jacentes.
AVIS CLIENTS
“La plupart des systèmes sont au même niveau en ce qui concerne les fonctionnalités. STARLIMS s’est distingué en proposant un système très ouvert avec une grande flexibilité et le meilleur mélange entre les fonctionnalités disponibles et la convivialité. De plus, nous avions confiance en leur équipe de projet.”
Alexandra Michel
Chef de projet, Bayer CropScience
“La solution LIMS est connectée à nos systèmes de production et de réception des matériaux au stockage. Cela signifie une avancée fondamentale dans les processus de contrôle de la qualité de l’entreprise, ainsi qu’un plus grand échange d’informations entre les systèmes.”
David Martínez Baños
Département informatique
“J’aime le fait qu’il soit convivial. J’aime le professionnalisme de tous mes interlocuteurs. J’apprécie également le fait qu’il s’agisse d’une amazon “STARLIMS gère de manière centralisée les échantillons et rationalise les processus de laboratoire associés pour nous permettre de fournir des données de qualité analytique en temps réel et a considérablement amélioré notre capacité à servir de point de contrôle de service pour tous les carburants aérospatiaux et les produits connexes pour les systèmes d’armement de l’AF dans le monde entier.”
Dave Fisher
Chef de la division laboratoire, Agence pétrolière de l'armée de l'air
“Le logiciel que nous utilisions auparavant était basé sur Microsoft Access. Avec toutes les limitations qu’il comportait, le principal problème auquel nous étions confrontés était l’impossibilité de l’interfacer avec d’autres logiciels (SAP, CRM, etc.).”
