We are pleased to announce the release of Starlims Technology Platform (TP) 12.6, which will deliver new features, an improved browser-based mobility experience, and optimizations that further elevate the capabilities and performance of our system.
How the Starlims Technology Platform Supports You
Starlims Technology Platform is the foundation for building and running laboratory business applications. It allows organizations to run their LIMS apps totally decoupled from the technology that supports them. As a modern technology system, the Starlims Technology Platform includes important components such as analytics, enterprise software integration support, and data manipulation support, as well as tools and frameworks for application development for both desktop and mobile-based devices.
TP 12.6: Improved Browser-Based Mobility
The flagship feature of TP 12.6 release is a new mobility framework that supports running mobile apps directly into the mobile browser available on your device.
When we introduced Starlims mobility more than 10 years ago, mobile browsers lacked the essential functionality required by an enterprise mobile app. As a result, we created the myStarlims native app that acted like a shell for all the mobile capabilities located in your Starlims system.
As mobile browser technology evolved, we decided to drop the myStarlims requirement and instead use the mobile browser directly to access the mobile capabilities of our LIMS.
Starting with TP 12.6, all your mobile apps developed using Starlims Designer or the Sencha libs will run potentially unchanged or with minimum modifications, directly in the mobile browser. This includes all advanced capabilities such as:
- Camera support
- QR barcode scanning
- Offline mode
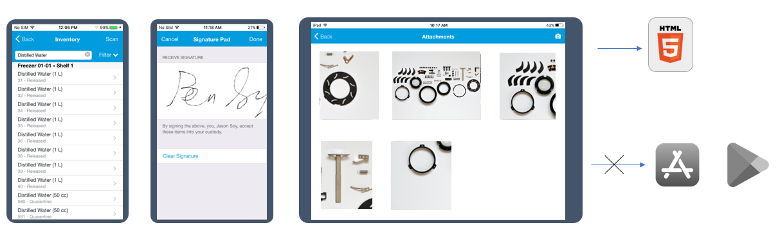
Image 1: Mobile apps running directly in mobile browser. No need to download myStarlims from public stores
We are confident that this change in our mobility solution will ease the burden on IT departments to deploy and update the myStarlims app on mobile devices. The change will also enable you to run the mobile capabilities on a wider range of mobile devices if they contain a compatible HTML5 mobile browser.
Please refer to the included documentation for more details about the transition to new browser-based mobility.
Additional Enhancements
Besides mobility, TP 12.6 also contains numerous other enhancements that optimize product functionality, performance, stability, and security.
TP 12.6 is a general technology release that is recommended to all Starlims v11/ v12 customers. We always recommend upgrading the Technology Platform as often as possible in order to keep your Starlims system in top shape, and to stay on top of changes in the IT landscape.
It is our mission to provide you with the best tools and support to keep your laboratory operations running as smoothly and effectively as possible.
Conclusion
We are confident that the new mobility solution introduced by this Technology Platform release will help you better achieve your mobility goals. The Starlims team is always here to help with proper operation and adoption of our new technology, and we look forward to your feedback!

