Delivering quality, safe, and compliant products is critical in manufacturing, but economic, regulatory, and technological pressures are making it hard for companies to bring goods to market faster without compromising quality or increasing spend. What if you could streamline quality operations, improve workflow inefficiencies, and increase productivity with one unified system?
Starlims is the only vendor that has a truly unified manufacturing informatics solution that allows you to accelerate time to market with less overhead.
The Starlims Quality Manufacturing Informatics Platform simplifies complex processes, easily integrates with other tools and systems, and extends data collection beyond the lab with one system, and one partner.
Request a Demo
Your Challenges

Operational Inefficiencies
Complicated workflows, manual processes, and siloed data and systems increase the risk for error and time to complete tasks.

Productivity Issues
A diminishing labor pool and lack of transparency across vendor/organization relationships slows market growth and expansion.

Rigid Compliance Requirements
Stringent and/or new regulatory compliance requirements add pressure to an already complex industry.

Speed to Market Delays
Rising costs, supply chain disruptions, and siloed business units/corporate functions make it costly to manufacture, store, and ship drugs.
Our Solution
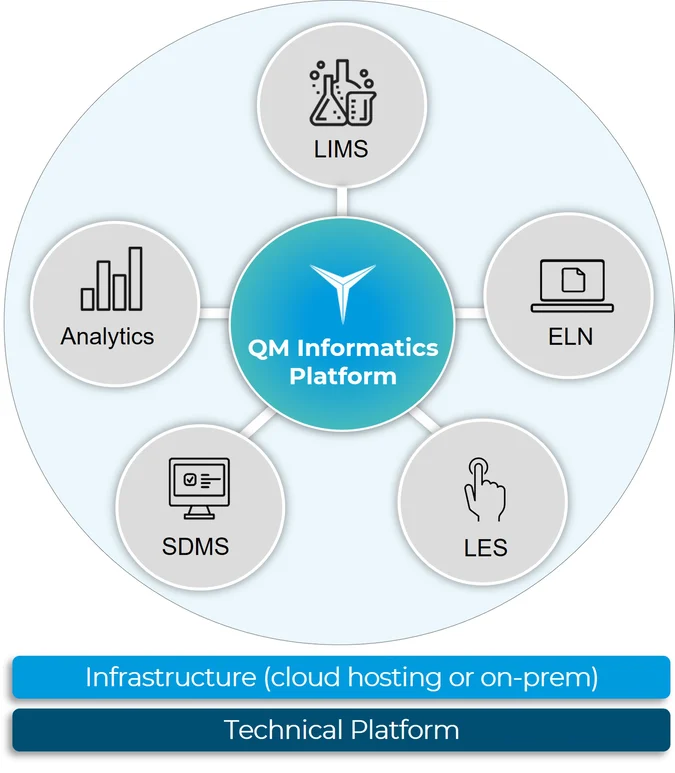
The Starlims Quality Manufacturing Informatics Platform
With countless systems, complex workflows, and large amounts of data, managing quality control processes in manufacturing is difficult. It’s crucial for organizations to have a unified system that can streamline processes and improve efficiencies, while also providing a holistic view of quality control and lab testing operations.
The Starlims Quality Manufacturing Informatics Platform seamlessly integrates the LIMS with the SDMS, LES, ELN, and Advanced Analytics solutions to meet your quality control and manufacturing needs in and outside the lab. Learn more in our Solution Brief.







