
A Cost-Effective SaaS LIMS with Less Complexity
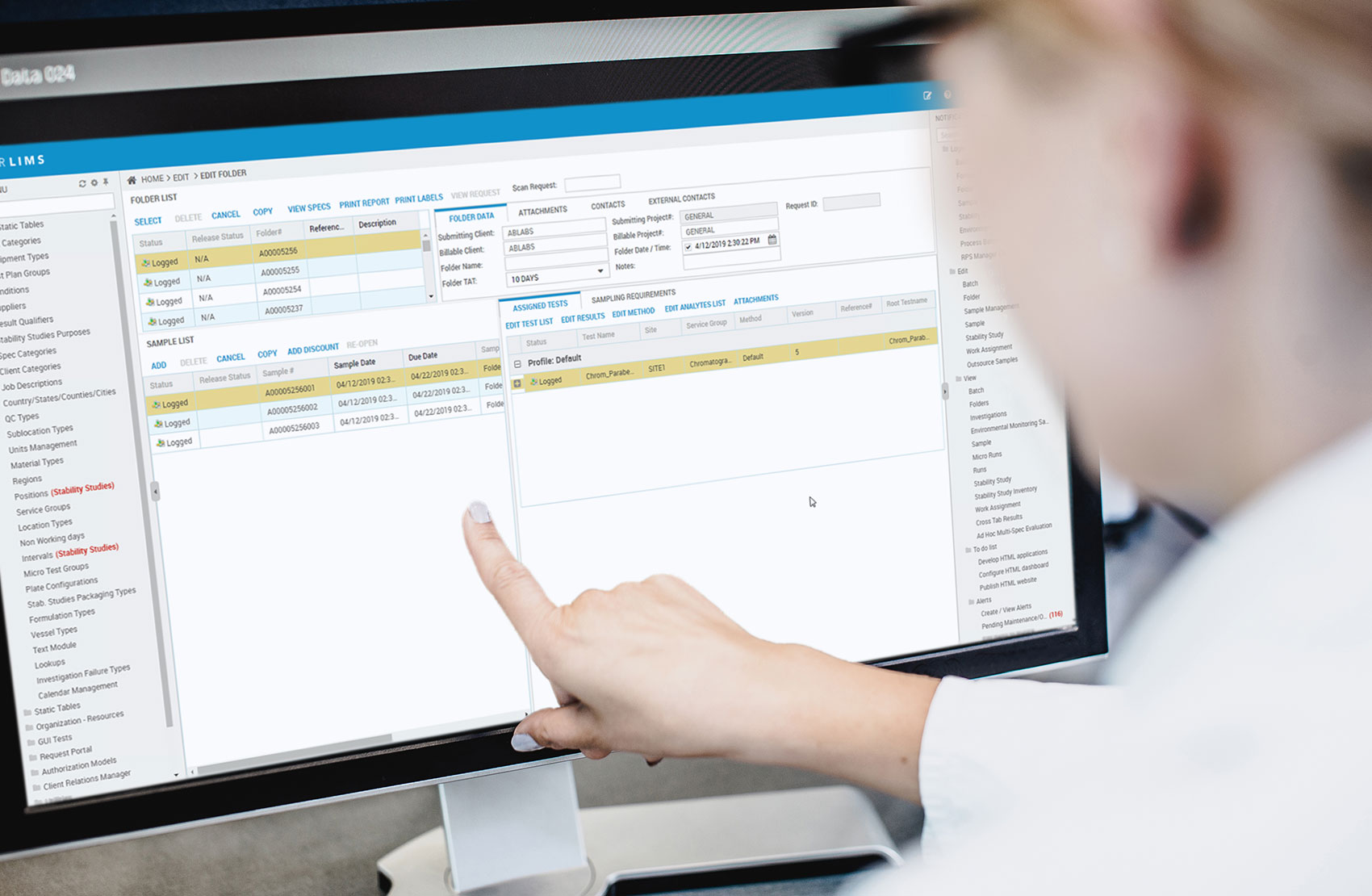
Pre-configured and pre-loaded with the critical features like batch login, environmental monitoring, outsource samples, and static data, The QM Essentials LIMS is a subscription-based solution that allows pharmaceutical, food & beverage, and consumer goods manufacturing labs to get to market faster without breaking the bank. Less complexity means less time to validate and activate your lab, and with a cloud-based subscription, it’s a lower upfront cost that helps SMB batch manufacturing labs save time, money, and resources.

Start Fast and Grow as You Go
With QM Essentials, you can start with what you need now and scale the QM Essentials LIMS as your organization grows. STARLIMS’ cloud-based environment is purposefully designed for quicker implementation and deployment, coming pre-configured with all the necessary workflows so that your lab can get started fast. Based on our Quality Manufacturing LIMS 12.5 technology, QM Essentials offers organizations a less complex, cost-effective solution that fits your lab’s current requirements, but is built with the ability to grow into a more comprehensive solution in the future.

What Comes With QM Essentials
Starting at a minimum of five users, QM Essentials is a subscription-only cloud offering pre-loaded with the most important features and static data necessary to quickly deploy and start using your LIMS. The workflows include Batch Login, Environmental Monitoring, and Outsource Samples (for CMO). If Stability Studies are critical for your manufacturing lab, you can easily add that on for an additional cost. QM Essentials also comes pre-configured with roles including QM Essential Admin, Lab Manager, Lab Analyst, Lab Assistant, Inventory Manager, Sample Reception, and Stability Study Manager. With STARLIMS support included in your package, you’ll have all the help you need to quickly and effectively launch QM Essentials!
Need Something More Comprehensive? Try Our QM LIMS
If your lab has more complex workflows and needs that extend beyond what QM Essentials offers, then our Quality Manufacturing LIMS (QM) is a better fit for your lab. Our robust LIMS offers more than 15 preconfigured workflows—ranging from batch testing with COA and stability studies to environmental monitoring, sample receipt, outsourced testing, continuous process support, and more. With access to hundreds of ready-to-use reports, you can begin tracking and improving your workflows for more streamlined laboratory operations. Plus, with full audit trails and electronic signatures, we help support you in reaching your compliance goals with confidence.
Resources

G2 Winter 2025: STARLIMS Excels in LIMS and Beyond
STARLIMS is recognized for excellence in Lab Informatics, according to customer reviews on G2.

LIMS Best Practices: Six Steps to Success
To truly harness the power of LIMS, you need to implement best practices that will unlock its full potential.

Newform Foods Shapes Tomorrow’s Food Landscape with Support from Labstep, a STARLIMS Company
Newform Foods has digitalized its R&D efforts with the Labstep ELN, increasing productivity, collaboration and knowledge sharing, and data security.

WEBINAR | Driving R&D Experiment Efficiency Through the User Experience
Driving experiment efficiency starts with a better user experience. Learn how UX can simplify experiment execution and accelerate R&D.

Infographic: R&D Experiment Data Capture Checklist
A comprehensive experiment record isn’t just about collecting information. It’s about ensuring that data is actionable, traceable, and meaningful.

WEBINAR | Advancing R&D with Collaboration
Learn how R&D labs can fan the flames of innovation with collaboration and cross-discipline science.



