Get Results with Starlims
From accelerating discovery and streamlining quality control initiatives in life sciences, to optimizing lab processes and simplifying compliance, Starlims removes the inefficiencies that slow you down.

Accelerate Innovation to NPI
By simplifying the development of your test methods and accelerating transfer from R&D to quality control and manufacturing, we quickly help you take your ideas from concept through to production.

Expedite Batch Release
By breaking down departmental silos, integrating systems, and improving operational efficiencies, our chemical LIMS, ELN, LES, and SDMS software help you expedite batch release to get to market faster.

Increase Productivity
By optimizing lab resources and processes, automating repetitive tasks, simplifying testing procedures, and expanding your testing capacity, we help your organization grow while maintaining a manageable cost base.

Simplify Compliance & Reduce Risk
Whether it’s for regulatory, customer, or corporate compliance, we help you capture, automate, track, and report on the correct data, processes, and evidence that enable you to reach your compliance goals.

The STARLIMS Life Sciences LIMS for Manufacturing
The STARLIMS Life Sciences LIMS, helps manufacturers leverage their lab data to drive success.
Automate workflows, minimize manual effort, and improve productivity in the lab with our LIMS. With functionality built specifically for the biotech and pharmaceutical industries, our LIMS helps lab leaders and teams around the globe simplify compliance and gain comprehensive oversight of laboratory operations.
All Your Lab Informatics in One Platform
As a pharmaceutical or biotech manufacturer, it’s critical that your products are safe, high quality, and effective. That’s hard to achieve if your time is focused on manually running a lab, instead of bringing innovations to market.
By combining your chemical LIMS with our ELN, SDMS, LES, and Advanced Analytics solutions, you can get a high level view of your lab’s data activity in real time
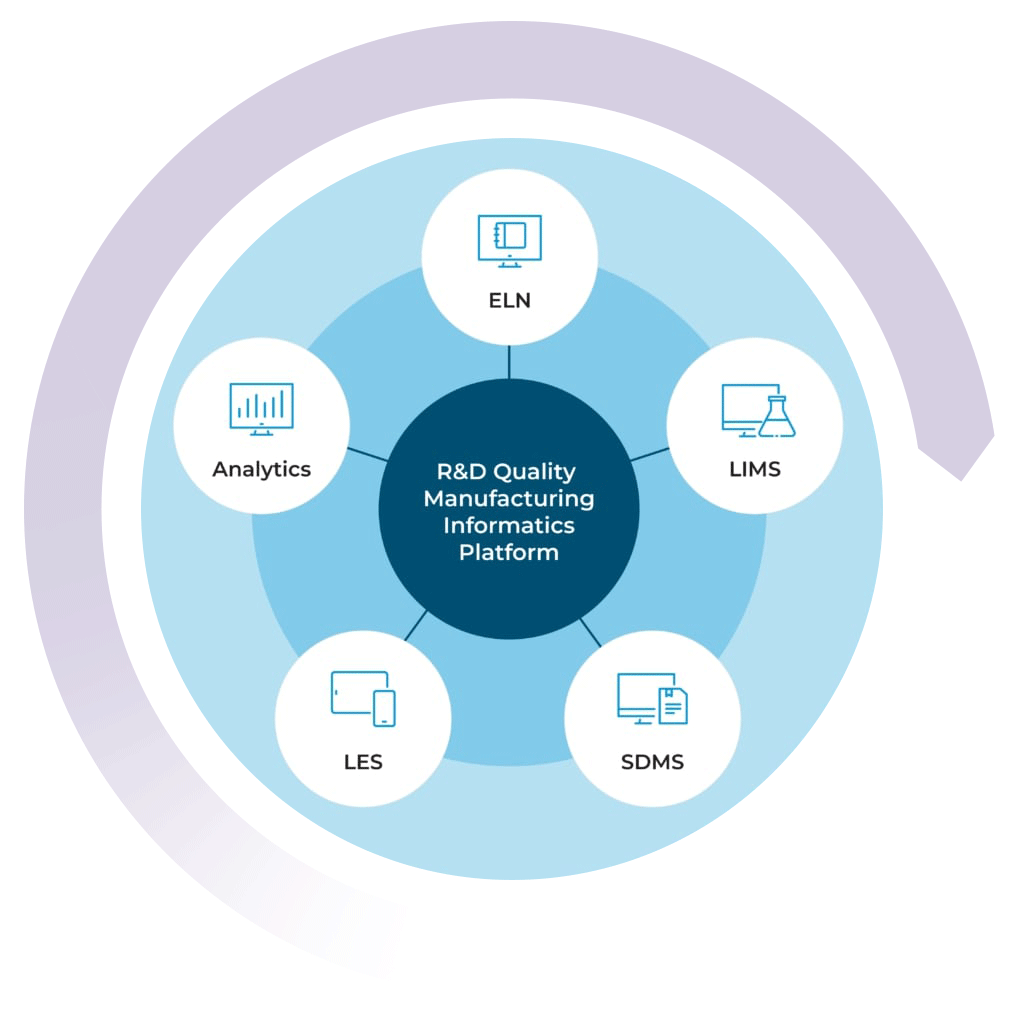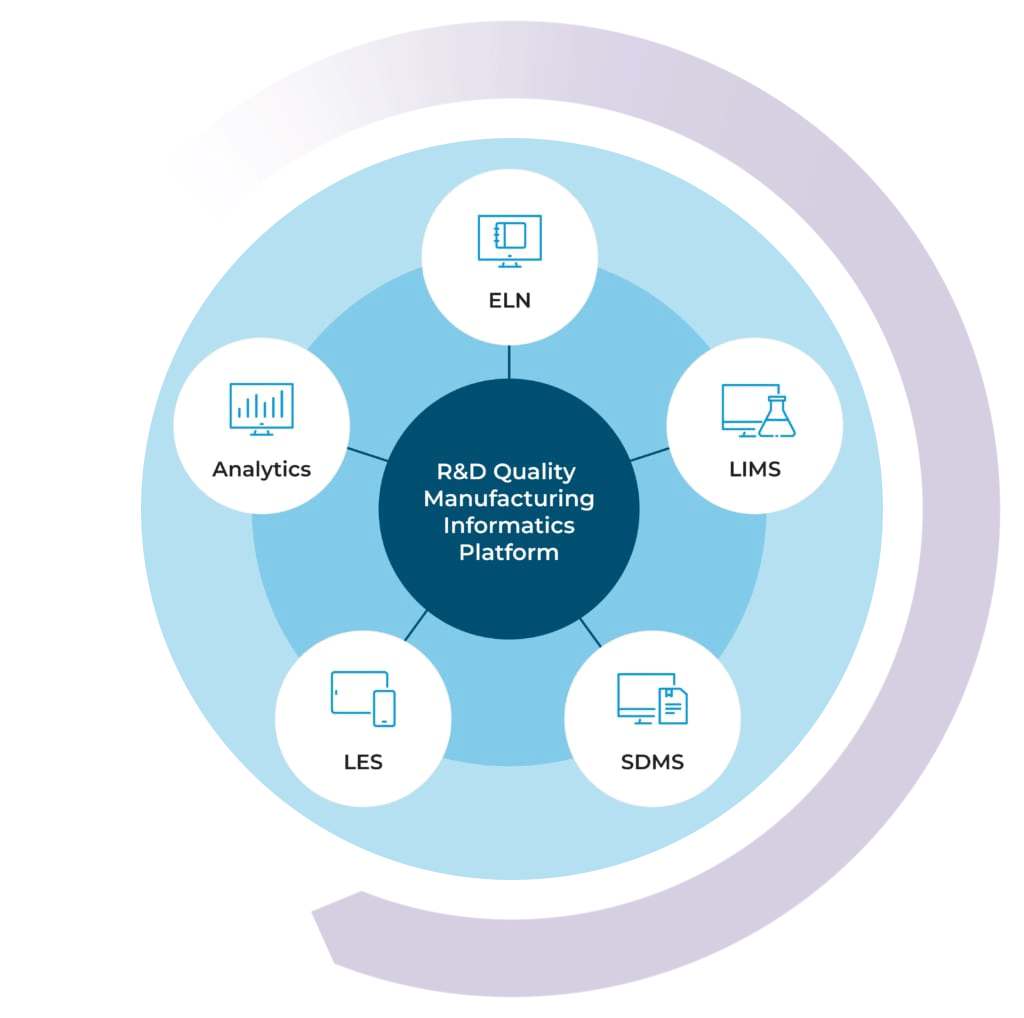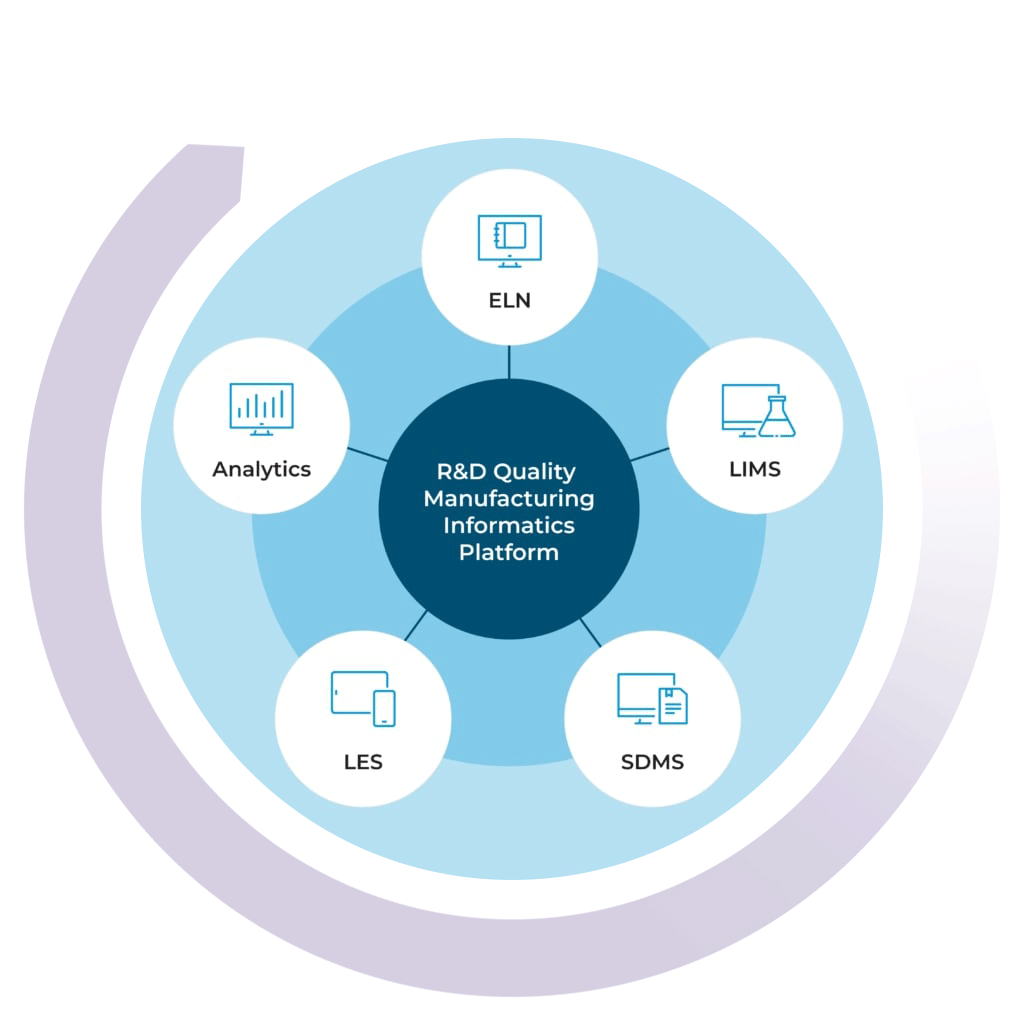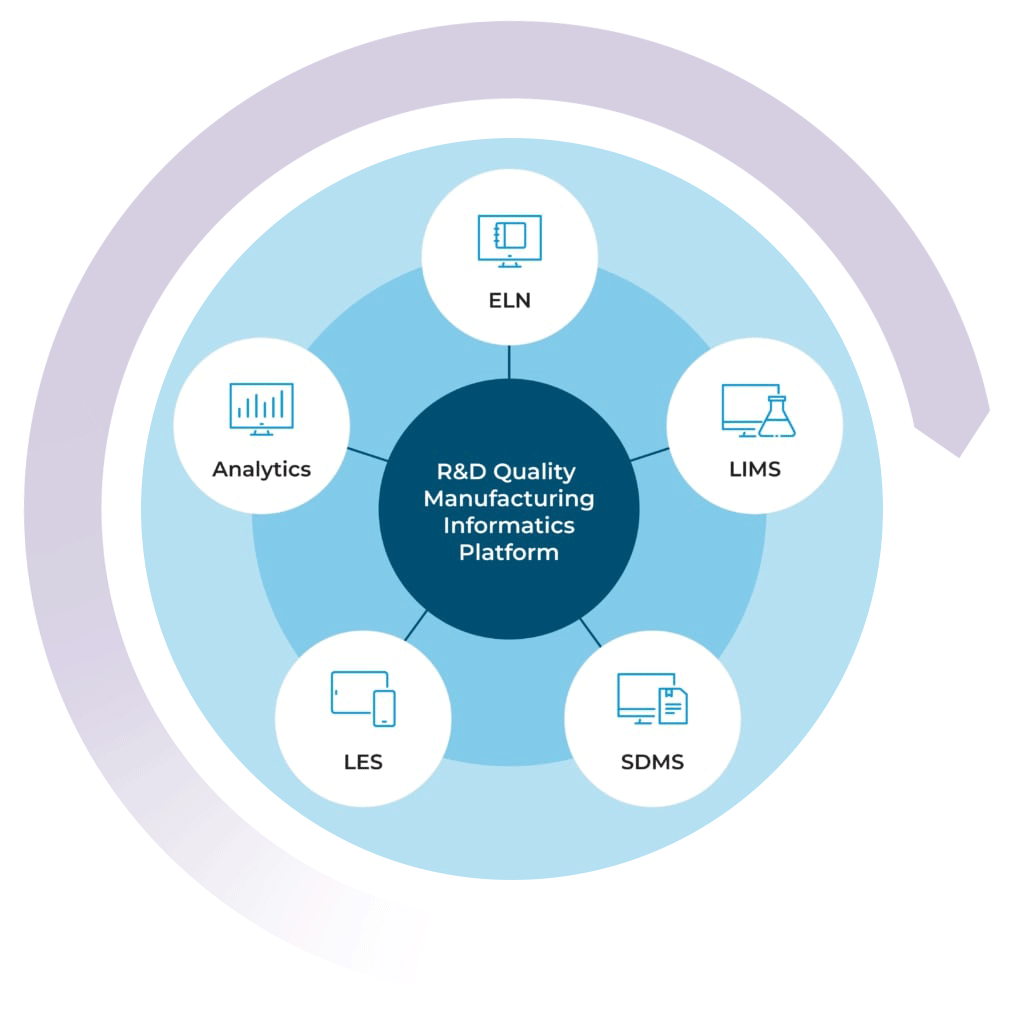
The R&D Quality Manufacturing Informatics Platform

From managing the discovery process and meeting compliance requirements, to navigating operational inefficiencies and speed-to-market delays, there are many hurdles to overcome when bringing drugs, therapies, and devices to market.
With the R&D Quality Manufacturing Informatics Platform, you can streamline processes, improve efficiencies, simplify compliance, and scale your business with one complete system.
Trusted by Our Customers
Resources

G2 Winter 2025: STARLIMS Excels in LIMS and Beyond
STARLIMS is recognized for excellence in Lab Informatics, according to customer reviews on G2.

LIMS Best Practices: Six Steps to Success
To truly harness the power of LIMS, you need to implement best practices that will unlock its full potential.

How To Traverse the Valley of Death in R&D
Hear from a Labstep customer how scientific collaboration helps R&D teams bridge the valley of death when it comes to discovery and innovation.

STARLIMS Celebrates This Fall as G2 LIMS, ELN, and SDMS Leader
STARLIMS is a leader in Lab Informatics, specifically for LIMS, SDMS, and ELN, according to reviews on G2.

What a LIMS is Not: A Review of Quality Software in Manufacturing
There are many software systems in a manufacturing environment, but many lack optimization for laboratory processes.

The Ultimate LIMS Playbook for Manufacturers
Read the Ultimate LIMS Playbook for Manufacturers. The definitive guide for mastering LIMS for manufacturing labs.




















