Get Results with Starlims
From sample management and monitoring, to automating lab processes and simplifying compliance, Starlims removes the inefficiencies that slow you down.

Improve Sample Management
We help you accurately collect, handle, and process specimens throughout their entire lifecycle by streamlining lab operations, improving data management, and closely monitoring progress.

Increase Productivity
By optimizing lab resources and processes, automating repetitive tasks, simplifying testing procedures, and expanding your testing capacity, we help your organization grow while maintaining a manageable cost base.

Simplify Compliance & Reduce Risk
We help you reach your compliance goals by allowing you to capture, track, and report on the necessary data needed to meet regulatory requirements.

STARLIMS Public Health LIMS
The STARLIMS Public Health LIMS, helps labs streamline operations, so you can deliver accurate results that keep our communities ahead of health risks.
Automate processes, improve data management, and collect and handle samples throughout the entire specimen lifecycle with ease using our LIMS. With features built specifically for public health, our LIMS helps lab leaders and teams around the globe automate their labs and support compliance and best practices.
One Complete Informatics Platform
In public health, it’s vital that you have timely and accurate data in order to protect the health of your community, but that’s a challenge if you’re manually operating your labs.
By combining your public health LIMS with our ELN, SDMS, and Advanced Analytics solutions, you can seamlessly collect, handle, track, and process specimens throughout their entire lifecycle.
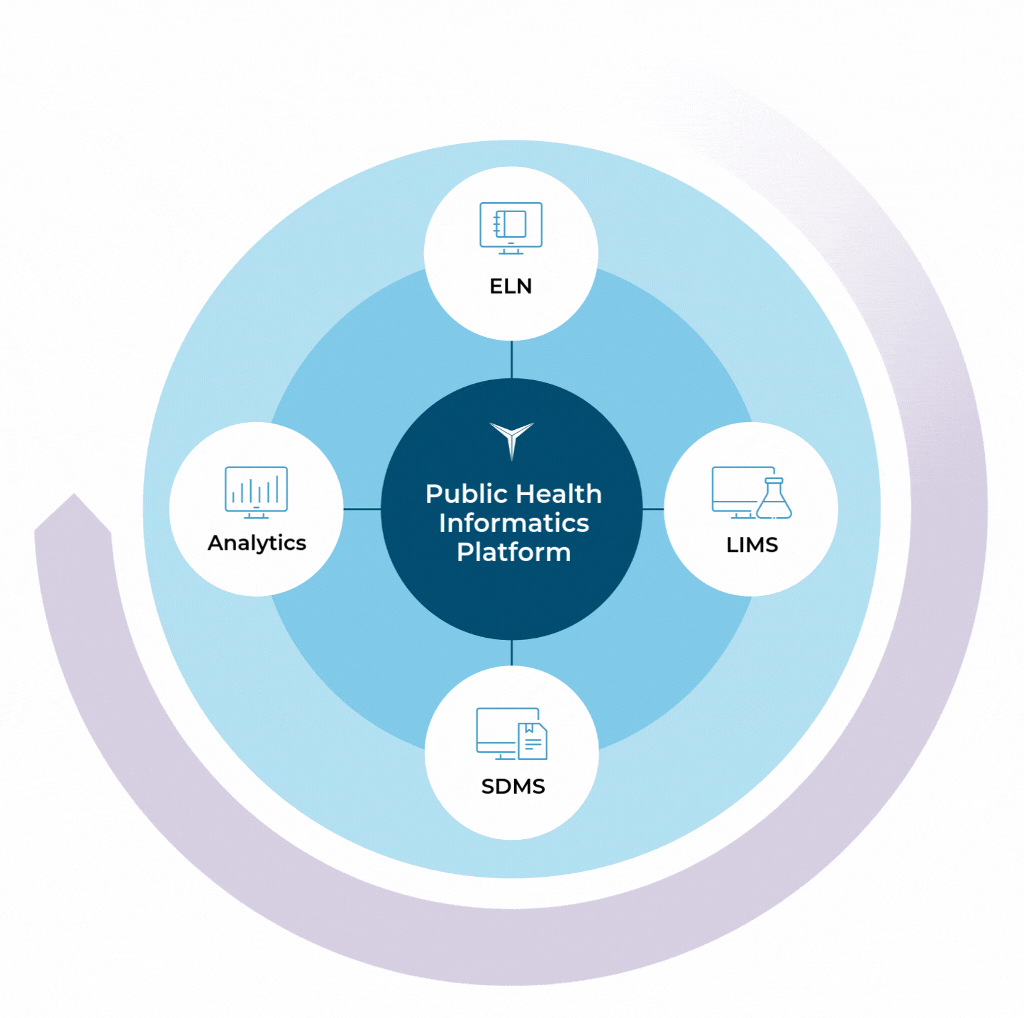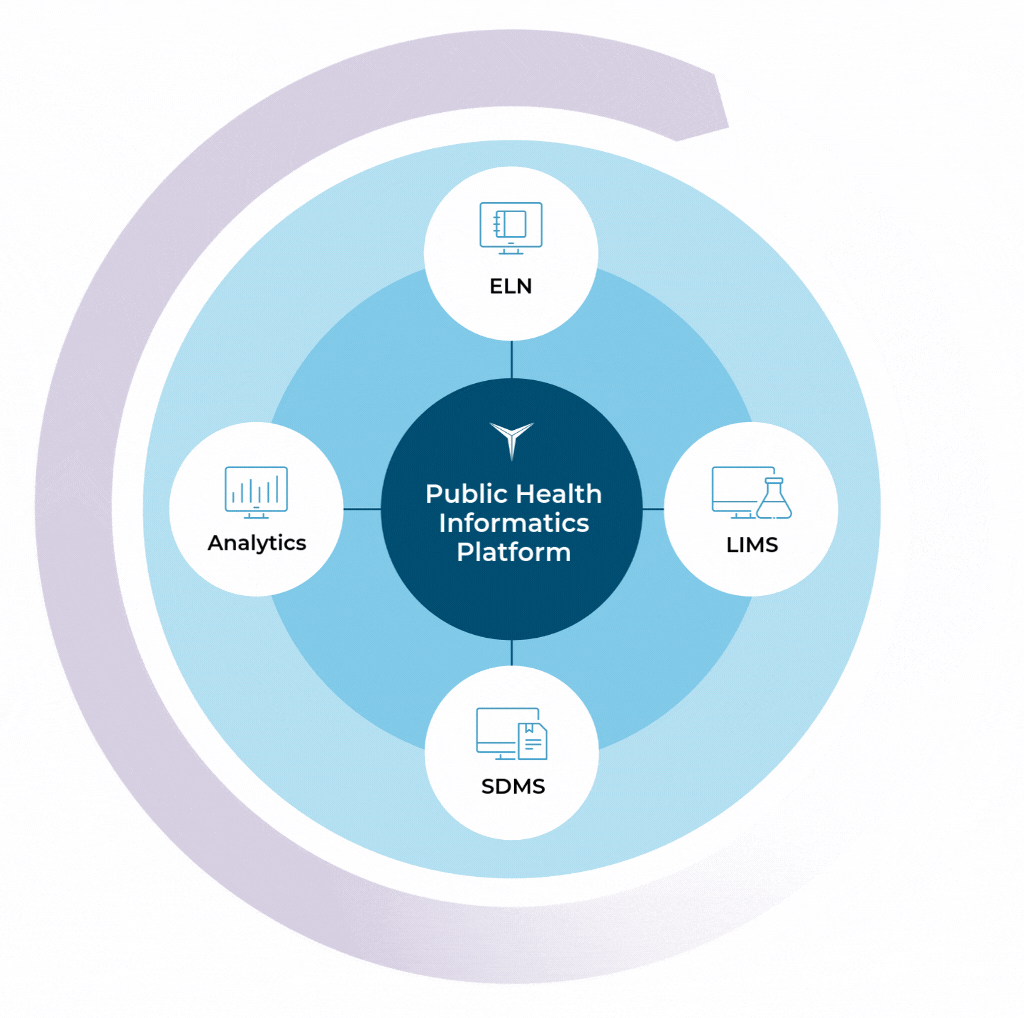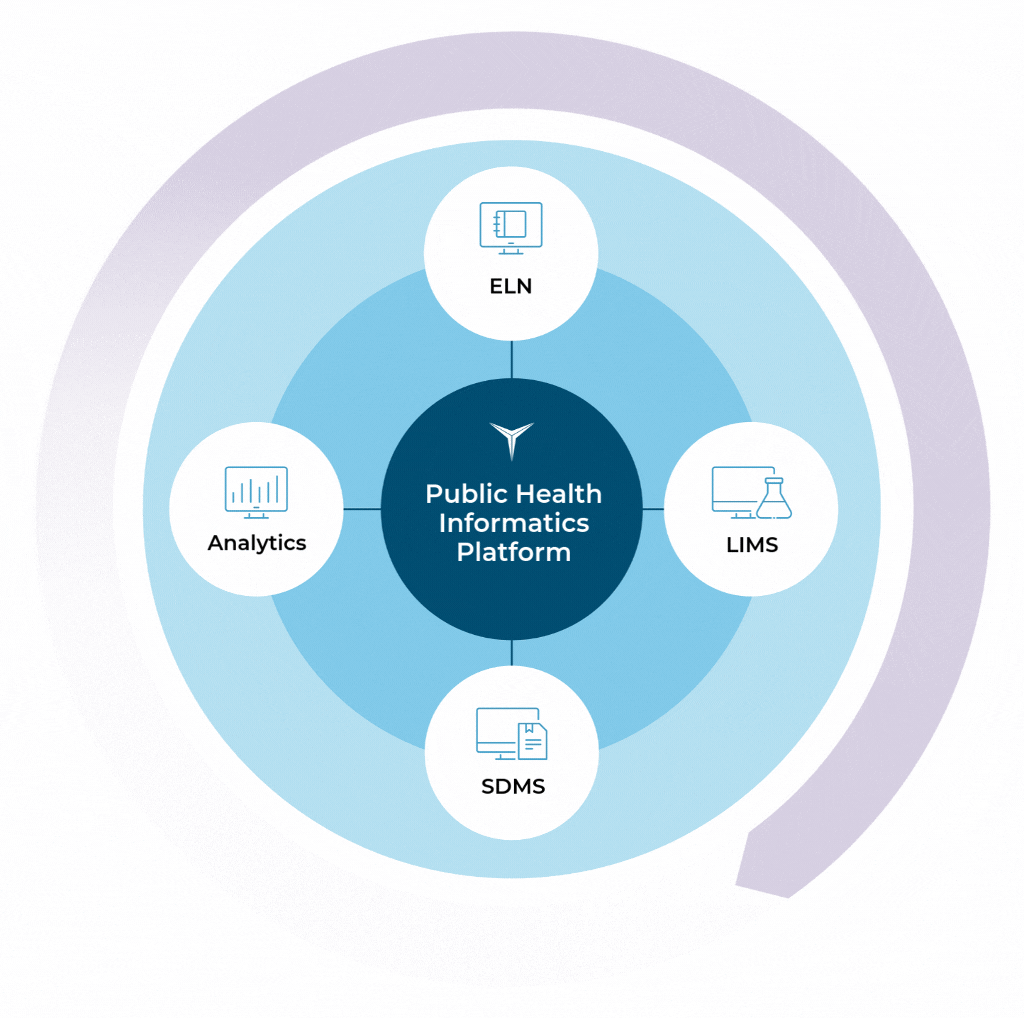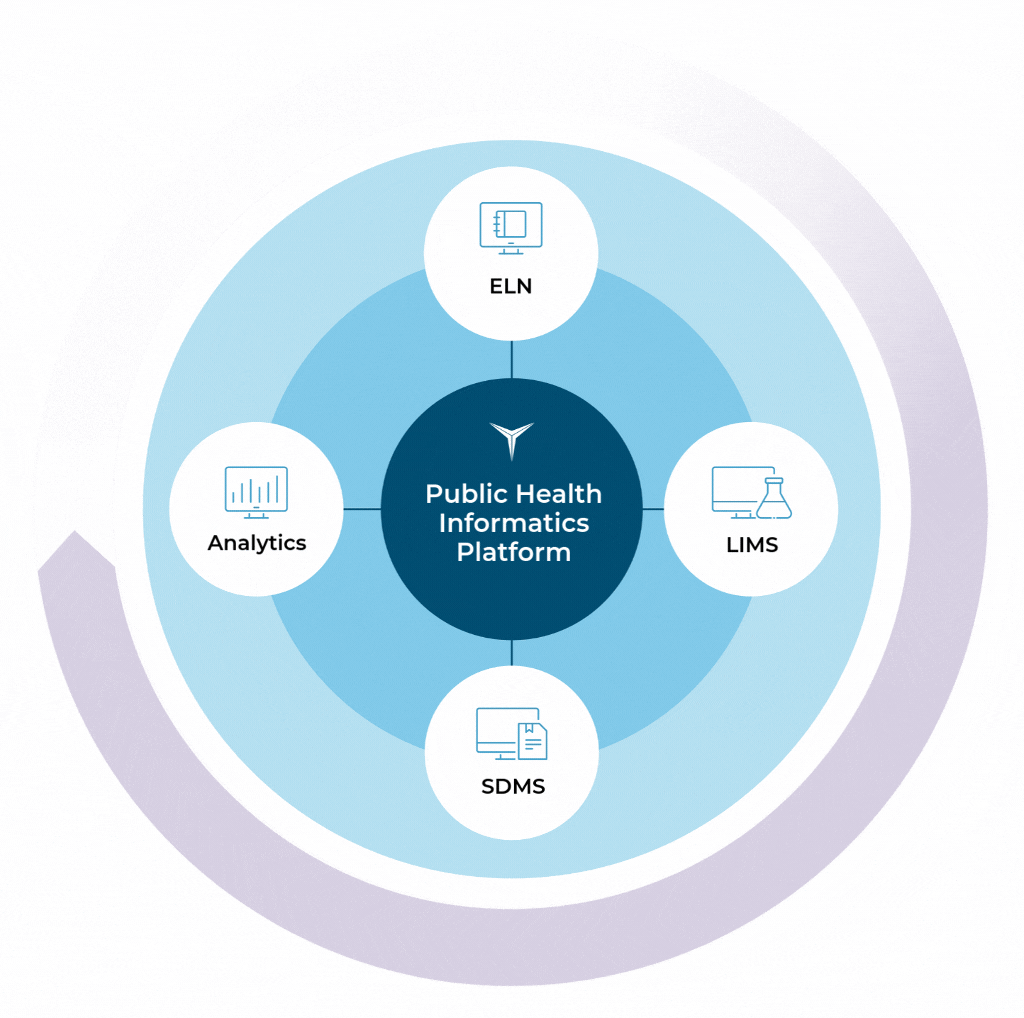
The Public Health
Informatics Platform

Take the complexity out of your laboratory operations with the Starlims Public Health Informatics Platform. Our LIMS, ELN, & SDMS software helps you collect, handle, and process specimens throughout their entire lifecycle for streamlined data collection and more accurate results.
From improved sample management and increased lab productivity, to better reporting and simplified compliance, learn how Starlims helps public health laboratories deliver the accurate results that protect our global population.
Trusted by Our Customers
Resources

The Public Health Informatics Platform Solution Brief
Public health officials depend on their labs’ abilities to not only monitor and track possible pandemics and epidemics, but to also determine causative effects whenever they occur. To do so, public health laboratories need the most secure, comprehensive, and compliant systems possible to keep agencies ahead of health risks and protect populations around the globe.

What’s New with Life Sciences for Public Health (LPH) 1.1
We’re excited to share the next phase in our multi-year roadmap – LPH 1.1!

G2 Winter 2025: STARLIMS Excels in LIMS and Beyond
STARLIMS is recognized for excellence in Lab Informatics, according to customer reviews on G2.

STARLIMS Celebrates This Fall as G2 LIMS, ELN, and SDMS Leader
STARLIMS is a leader in Lab Informatics, specifically for LIMS, SDMS, and ELN, according to reviews on G2.

STARLIMS Celebrates Year as G2 Leader in Lab Informatics
STARLIMS celebrates a year of leading G2 in Lab Informatics – accolades for ELN, LIMS, and SDMS.

STARLIMS Spends 4 Quarters as G2 Leader in Lab Informatics
STARLIMS has maintained its position as G2 leading Lab Informatics provider for four consecutive quarters, leading in ELN, LIMS, and SDMS.




















