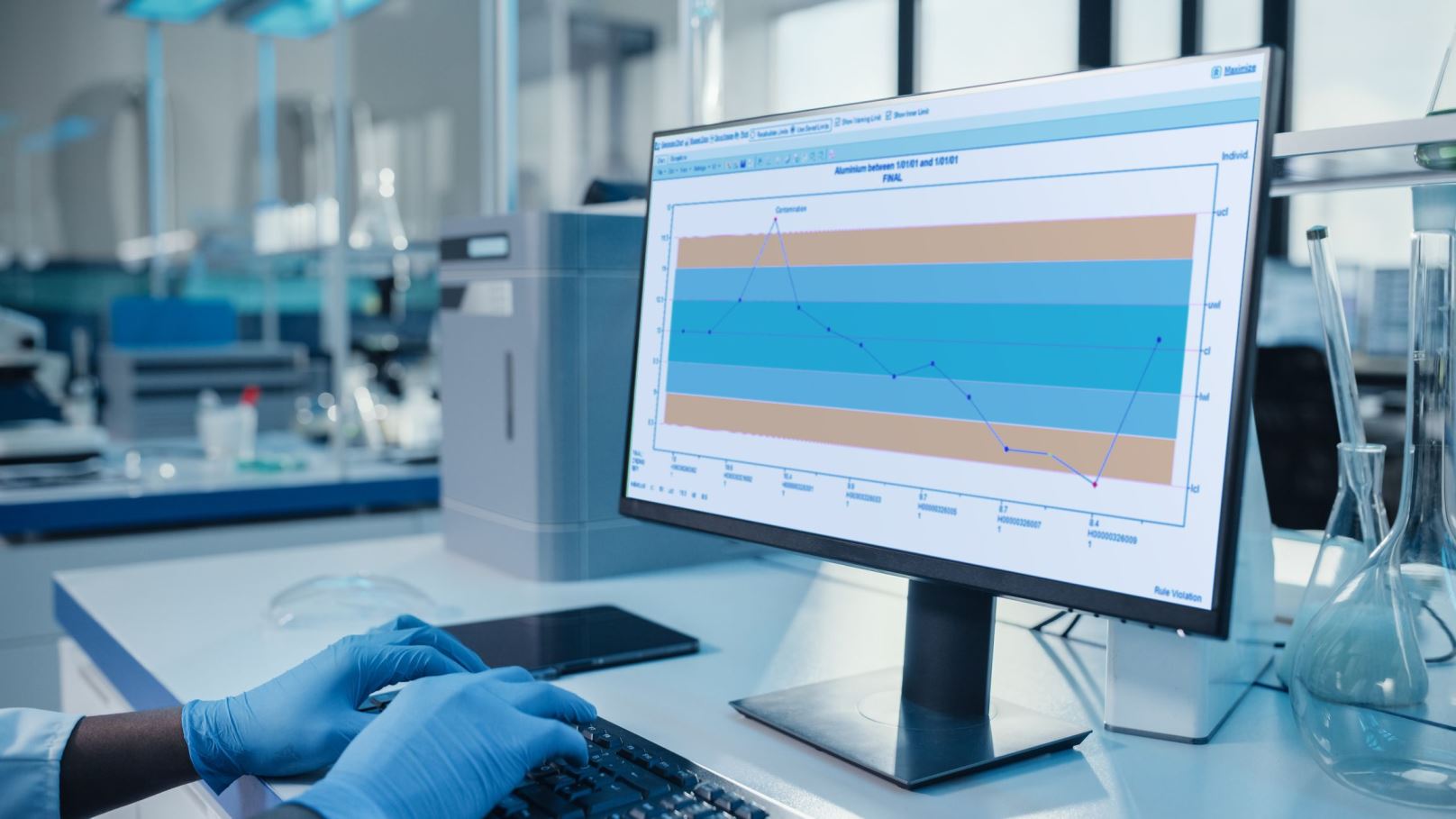
A Powerful, Comprehensive LIMS
Standardize and accelerate your lab processes with the Starlims LIMS. Our powerful, comprehensive Laboratory Information Management System contains over 15 OOTB workflows, including batch testing with COA, stability, environmental testing, materials receiving, contract labs testing, continuous process, sample testing, outsourcing, and more. With hundreds of OOTB reports, you can quickly start to monitor processes and workflows for better, more streamlined laboratory operations. And by providing full audit trails and electronic signatures, we also help you reach your compliance objectives.

Value-Added Solutions
In addition to our OOTB functionalities, our quality LIMS also provides a host of value-added solutions. From common laboratory equipment connectors and advanced SPC reporting with integration into NWA, to an advanced test method development and execution calculation engine, we support the needs of your business. As the only certified SAP connector, we also help you easily integrate other solutions into your ecosystem. And with our Request Management Portal, you have the ability to create and submit test requests to your contractors.

Globally Recognized LIMS
Our advanced laboratory information management system is the cornerstone of superior laboratory management technology, helping your lab operate at the pinnacle of efficiency, quality, and speed. Designed to quickly transfer from research to the rigorous environments of quality control and manufacturing, our LIMS helps you accelerate innovation to New Product Introduction, expedite batch release, and increase productivity in the lab.
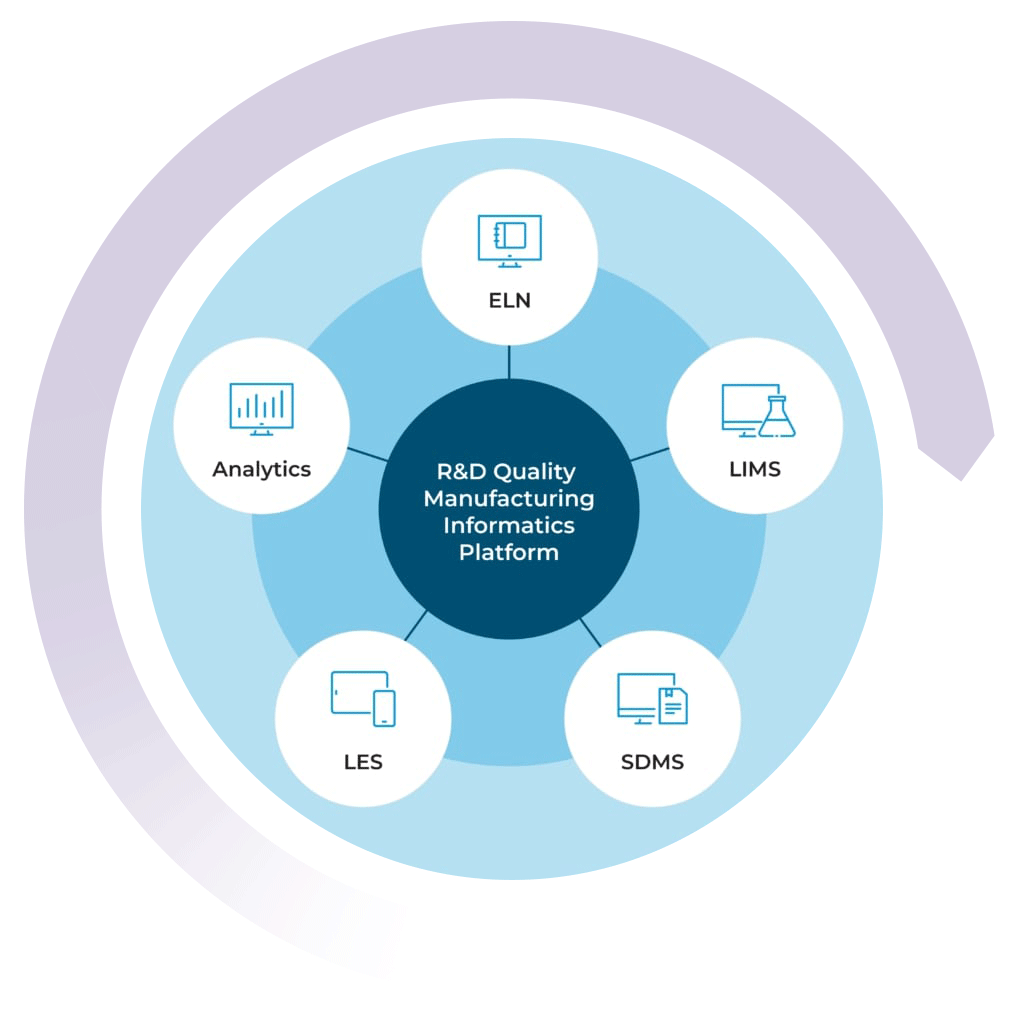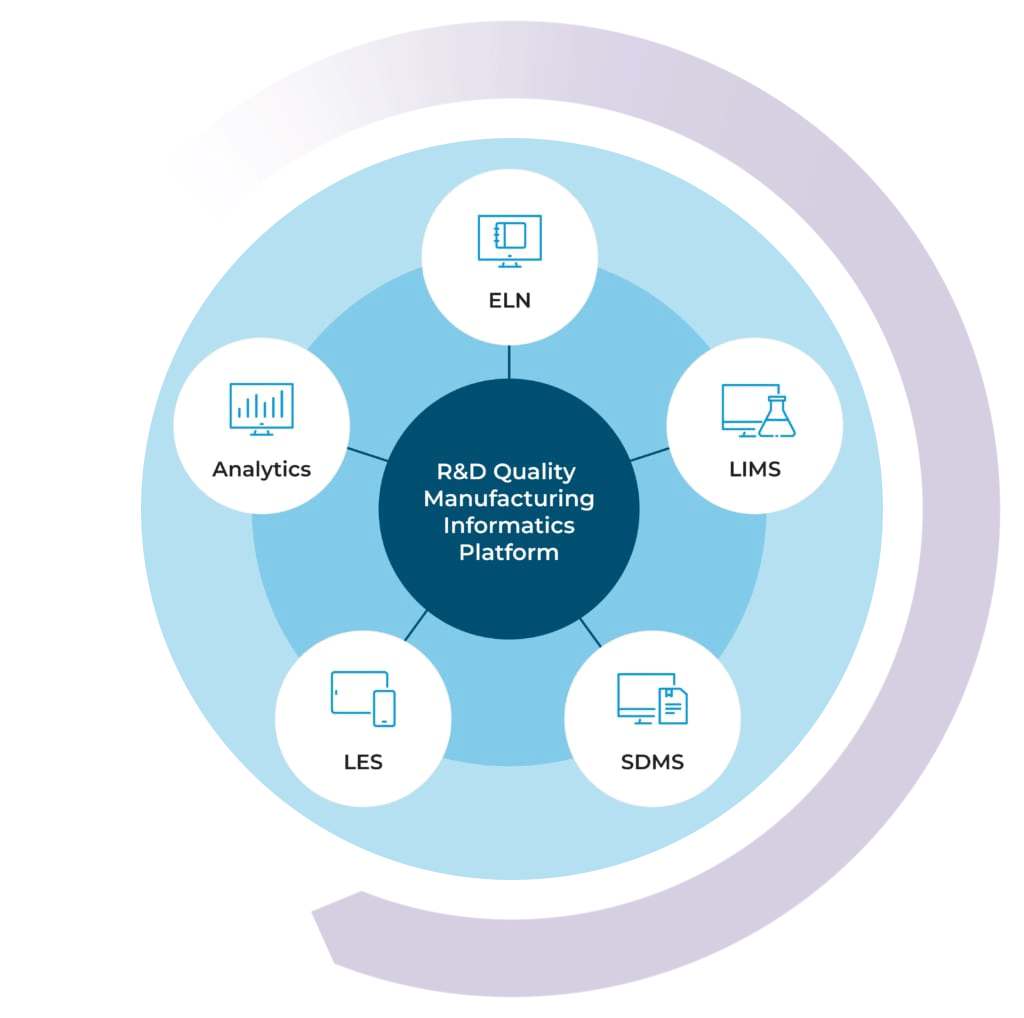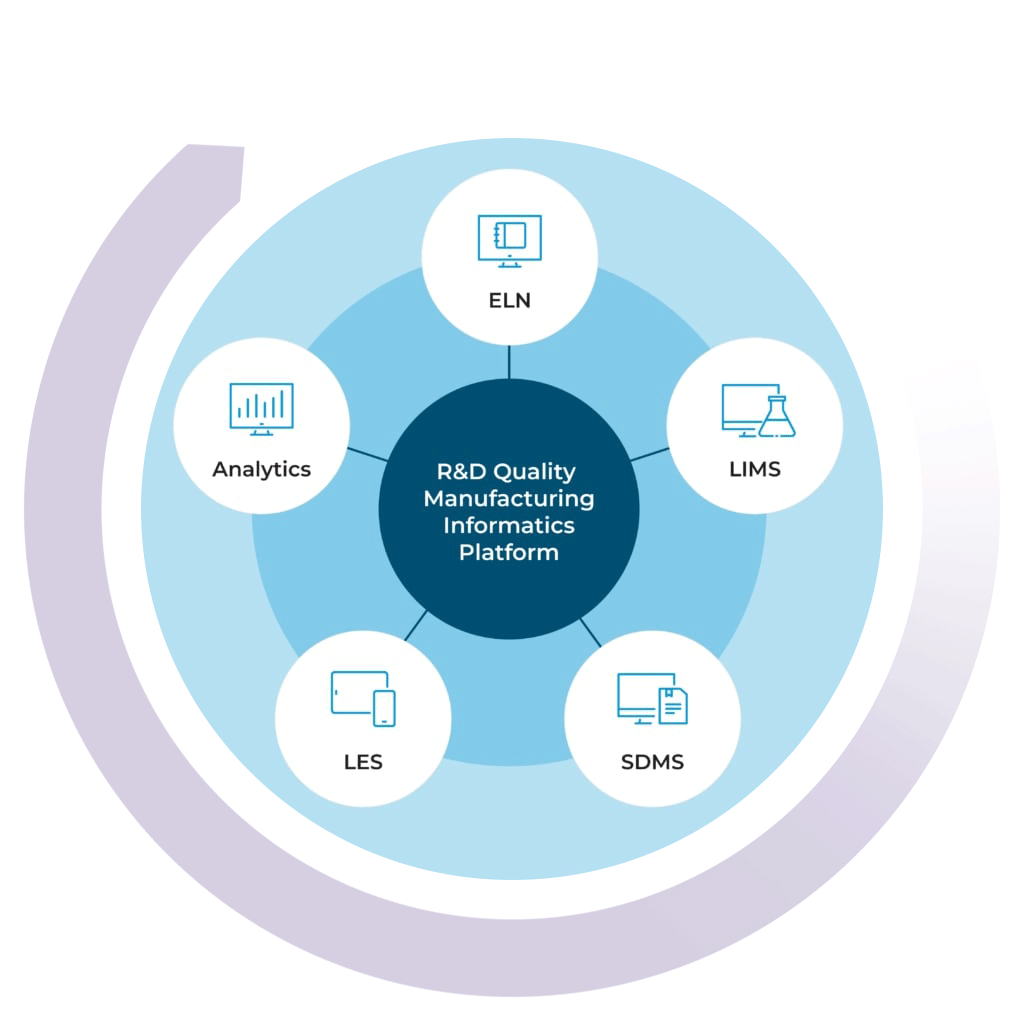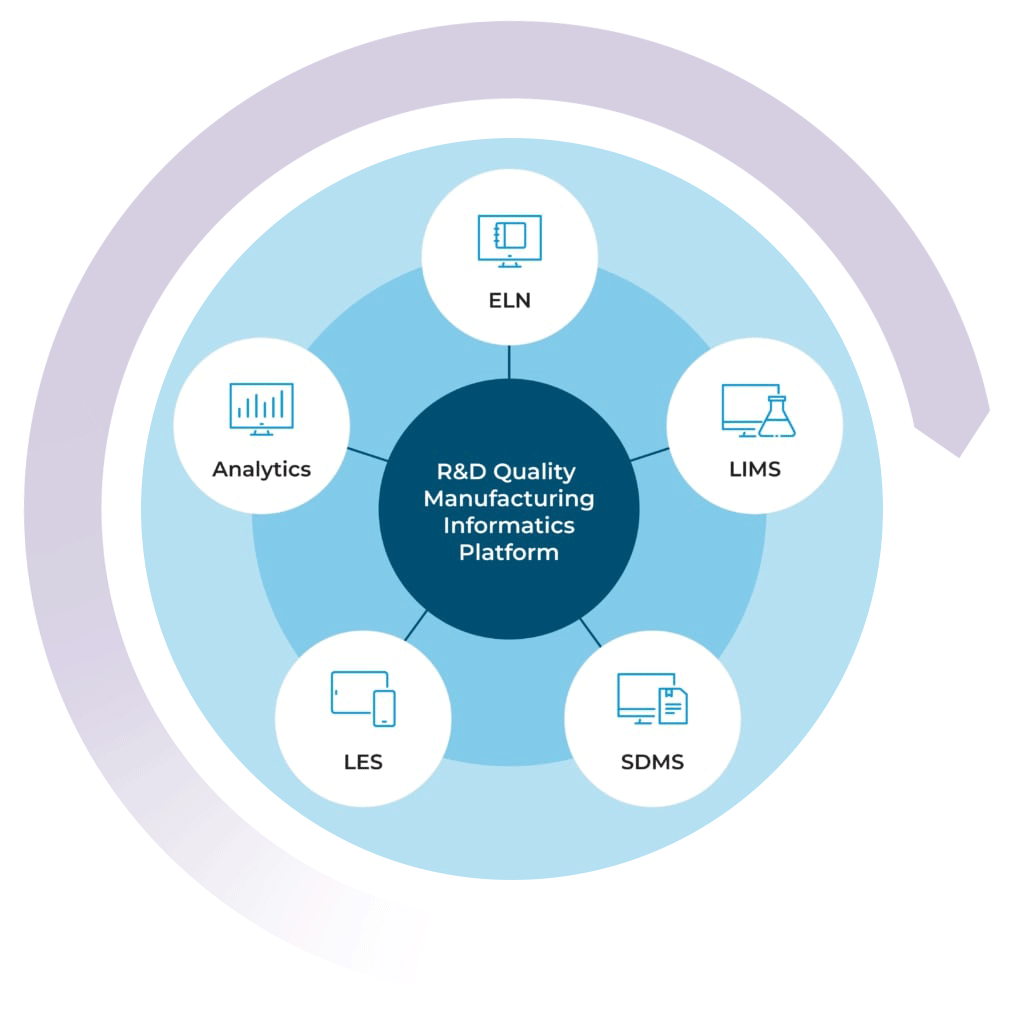
Building Your Lab Data Ecosystem
By combining the LIMS with the STARLIMS’ ELN, SDMS, LES, and Advanced Analytics solutions, you can get a 360-degree view of your lab’s data ecosystem. Push the boundaries of what your lab is capable of and uphold the highest standards of quality with the STARLIMS R&D Quality Manufacturing Informatics Platform.

See Our LIMS in Action
Choose the STARLIMS LIMS to achieve unparalleled laboratory excellence. Experience how our LIMS can transform your laboratory operations, driving your organization to new heights of success and efficiency.
Resources

G2 Winter 2025: STARLIMS Excels in LIMS and Beyond
STARLIMS is recognized for excellence in Lab Informatics, according to customer reviews on G2.

LIMS Best Practices: Six Steps to Success
To truly harness the power of LIMS, you need to implement best practices that will unlock its full potential.

Newform Foods Shapes Tomorrow’s Food Landscape with Support from Labstep, a STARLIMS Company
Newform Foods has digitalized its R&D efforts with the Labstep ELN, increasing productivity, collaboration and knowledge sharing, and data security.

WEBINAR | Driving R&D Experiment Efficiency Through the User Experience
Driving experiment efficiency starts with a better user experience. Learn how UX can simplify experiment execution and accelerate R&D.

Infographic: R&D Experiment Data Capture Checklist
A comprehensive experiment record isn’t just about collecting information. It’s about ensuring that data is actionable, traceable, and meaningful.

WEBINAR | Advancing R&D with Collaboration
Learn how R&D labs can fan the flames of innovation with collaboration and cross-discipline science.
